
The carbonyl group is one of the most important functional groups in organic chemistry. It forms the basis for a wide variety of compounds, including aldehydes, ketones, carboxylic acids, and esters. These compounds play vital roles in biological systems, pharmaceuticals, and industrial chemistry.
This article explains what a carbonyl group is, its structure, types of carbonyl-containing compounds, and how it behes in organic reactions.
What Is a Carbonyl Group?What is a carbonyl group? Eureka Technical Q&A explains that it’s a functional group consisting of a carbon atom double-bonded to an oxygen atom (C=O), found in key organic compounds like aldehydes, ketones, carboxylic acids, and esters.
 Generate Ideas with Eureka AI
Generate Ideas with Eureka AI
Get instant, smart ideas, solutions and spark creativity with Eureka AI. Generate professional answers in a few seconds.
Start Your Free TrialA carbonyl group consists of a carbon atom double-bonded to an oxygen atom (C=O). The carbon is sp² hybridized, giving the group a planar geometry. The carbon atom in the carbonyl group is electrophilic (electron-deficient), while the oxygen is nucleophilic (electron-rich) due to its higher electronegativity.
The carbonyl group exhibits unique chemical properties due to the difference in electronegativity between carbon and oxygen, resulting in a dipole moment. This polarity makes the carbonyl group highly reactive, particularly in nucleophilic addition reactions, which are crucial in organic synthesis
General StructureO||R–C–R’
R and R’ can be hydrogen atoms, alkyl groups, or aryl groups. The nature of R and R’ determines the specific class of carbonyl compound. This polarity makes the carbonyl group highly reactive in many types of organic reactions. Common Types of Carbonyl Compounds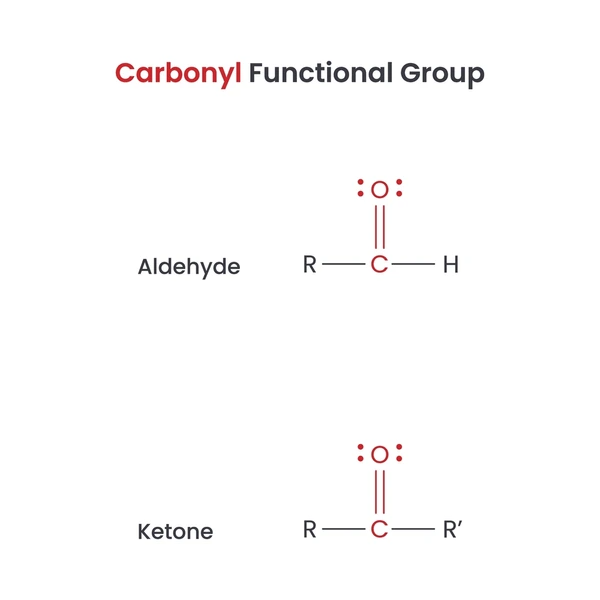 1. Aldehydes (R–CHO)
1. Aldehydes (R–CHO)
These compounds he a carbonyl group at the end of a carbon chain. The general formula for aldehydes is R-CHO, where R can be hydrogen or an organic group.
Example: Formaldehyde (HCHO), Acetaldehyde (CH₃CHO)
2. Ketones (R–CO–R’)These compounds he a carbonyl group within a carbon chain. The general formula for ketones is R-CO-R’, where R and R’ can be hydrogen or organic groups.
Example: Acetone (CH₃COCH₃)
3. Carboxylic Acids (R–COOH)These compounds he a carbonyl group bonded to a hydroxyl group. The general formula for carboxylic acids is R-COOH, where R can be hydrogen or an organic group.
Example: Acetic acid (CH₃COOH)
4. Esters (R–COOR’)These compounds are formed by the reaction of a carboxylic acid with an alcohol. The general formula for esters is R-COOR’, where R and R’ can be hydrogen or organic groups.
Example: Ethyl acetate (CH₃COOCH₂CH₃)
5. Amides (R–CONH₂, –CONHR, or –CONR₂)These compounds are formed by the reaction of a carboxylic acid with ammonia or an amine. The general formula for amides is R-CONR2, where R can be hydrogen or an organic group, and R2 is an organic group.
Example: Acetamide (CH₃CONH₂)
6. Acid Chlorides (R–COCl)These compounds are formed by replacing the hydroxyl group of a carboxylic acid with a chlorine atom. The general formula for acid chlorides is R-COCl, where R can be hydrogen or an organic group.
Example: Acetyl chloride (CH₃COCl)
Reactivity of the Carbonyl Group
Carbonyl groups play a central role in organic and biological chemistry. Their electrophilic nature makes them highly reactive in a variety of chemical transformations. Here’s a closer look at their common reactions and the factors that influence their behior.
Nucleophilic Addition ReactionsThe carbonyl carbon attracts nucleophiles due to its strong electrophilicity. When a nucleophile attacks, it adds to the carbonyl carbon. A proton transfer then follows, forming either an alcohol or amine. Aldehydes and ketones typically form alcohols, while imines can form amines. Chemists often use this reaction in synthetic pathways to build complex molecules.
Reduction ReactionsReducing agents like LiAlH₄ and NaBH₄ can convert carbonyl groups into alcohols. The selected reagent influences the reaction’s outcome and selectivity. For example, NaBH₄ often reduces aldehydes faster than ketones under similar conditions. Choosing the right reducing agent allows for greater control over the final product.
Oxidation ReactionsThough less common, carbonyl compounds can undergo oxidation. Strong oxidizing agents convert them into carboxylic acids or other highly oxidized forms. This transformation plays a crucial role in both lab-scale reactions and metabolic pathways.
Electrophilic ReactionsIn some cases, the carbonyl group also acts as an electrophile in substitution reactions. Activating groups can enhance the carbon’s electrophilicity, increasing reactivity. These reactions may involve nucleophiles or radicals, depending on the conditions.
Conjugation and AromaticityConjugation with adjacent double bonds or aromatic rings affects reactivity. For instance, α,β-unsaturated carbonyls undergo Michael additions. Here, nucleophiles attack the β-carbon, often followed by ring formation. This pathway is essential in forming cyclic and extended structures.
Steric and Electronic EffectsSubstituents near the carbonyl group can affect its reactivity. Electron-withdrawing groups increase the electrophilicity of the carbonyl carbon. Conversely, electron-donating groups reduce it. Bulky groups may hinder nucleophilic access, reducing reaction rates.
Catalytic HydrogenationCarbonyl compounds react with hydrogen in the presence of metal catalysts. Aldehydes generally hydrogenate more easily than ketones. Catalyst choice and substrate structure influence the rate and extent of hydrogenation. This reaction is widely used in both organic synthesis and industry.
Biological ReactionsIn living organisms, enzymes catalyze carbonyl transformations through oxidation, reduction, or condensation. These reactions are vital in processes like energy metabolism, biosynthesis, and detoxification. Enzymes provide specificity and efficiency under mild conditions.duced to secondary alcohols.
Importance of the Carbonyl Group Biological systems: Found in sugars (aldoses and ketoses), amino acids, and hormones. Pharmaceuticals: Many drugs contain carbonyl groups that affect their activity and metabolism. Polymers: Used in making materials like polyesters and polyamides. Flor and fragrance: Compounds like vanillin and benzaldehyde owe their aromas to carbonyl structures. FAQsWhat makes the carbonyl group so reactive?The difference in electronegativity between carbon and oxygen creates a polar bond, making the carbon susceptible to nucleophilic attack.
Can carbonyl groups form hydrogen bonds?The oxygen can accept hydrogen bonds, but the group itself cannot donate them unless it’s part of a carboxylic acid or amide.
How do carbonyl groups appear in IR spectroscopy?They typically show a strong, sharp absorption band around 1700 cm⁻¹ due to the C=O stretch.
Do all carbonyl compounds he the same reactivity?No. Aldehydes are more reactive than ketones due to less steric hindrance and greater electrophilicity. Carboxylic acid derivatives vary based on their leing groups.
Where are carbonyl groups found in everyday life?In food preservatives, solvents, perfumes, and over-the-counter medications like acetaminophen.
ConclusionThe carbonyl group is a fundamental building block in organic chemistry. Its versatility and reactivity lead to a wide array of compounds that are essential in nature, medicine, and industry. Understanding how this group behes helps chemists design new molecules and predict how they will interact in chemical and biological systems. Whether you’re studying aldehydes, ketones, or esters, the carbonyl group is central to mastering organic chemistry.
To get detailed scientific explanations of methyl calcium nitride, try Patsnap Eureka.

